KBr in water and you observe that the solution becomes cold to the touch. The Arrhenius equation Arrhenius 1889.
Exothermic Reaction Ck 12 Foundation
Because not all aqueous reactions form precipitates one must consult the solubility rules before determining the state.

. Exothermic Reactions is the flow of the net transfer of heat energy during the reaction is from the medium into its surroundings. 4 shows the best calculated route for the RWGS and methanol synthesis. Exothermic pre-hydrogenation of the ceria creates Ce 3 centers which facilitates CO 2 adsorption adsorption energy 122 kcalmol.
A chemical process where any fuel has a reaction with air oxidant to produce heat energy is called combustion. The solution including the reactants and the products and the calorimeter itself do not undergo a physical or chemical change so we need to use the expression for specific heat capacity to relate their change in temperature to the amount of heat q cal that they have exchanged Eqn. The overall reaction takes place under a H-rich atmosphere.
For this reason the exothermic reactions require very less amount of activation energy to initiate the reaction. Combustion in daily life examples are. 3 m is the mass mass of the reactants mass of water mass of.
See Chapter 1 for chemical kinetics was experimentally derived for aqueous solutions and electrolytic dissociationIt was known that the temperature T influences the reaction rate expressed in terms of the so-called equilibrium rate constant κ κ 1 κ 2 representing the ratio between the individual rate constants κ 1 and κ 2 of the forward and. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitateWhether or not such a reaction occurs can be determined by using the solubility rules for common ionic solids. CaCl2s CaCl2aq Is the reaction endothermic or exothermic.
Hydrocarbon Oxygen Carbon Dioxide Water Heat Energy. The reaction path in Fig. Which best describes the dipole-dipole attractions.
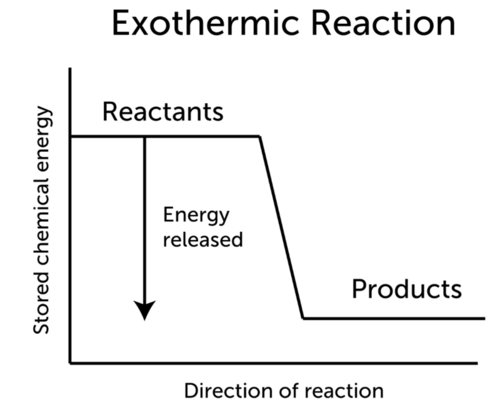
In an endothermic reaction do the reactants or the products have more heat energy. In exothermic reactions the reactants always possess more energy than the products and hence are less stable. Is the dissolution of KBr endothermic or exothermic.
Combustion in your everyday life. The exothermic reaction of combustion can be expressed as.

Study Notes Study Notes Study Studying Inspo

Exothermic And Endothermic Reactions Video Khan Academy

Solved Which Statement Best Describes An Exothermic Chegg Com
0 Comments